
Effective Catalytic Reduction of Methyl Orange Catalyzed by the Encapsulated Random Alloy Palladium‐Gold Nanoparticles Dendrimer. - Ilunga - 2017 - ChemistrySelect - Wiley Online Library

Effective Catalytic Reduction of Methyl Orange Catalyzed by the Encapsulated Random Alloy Palladium‐Gold Nanoparticles Dendrimer. - Ilunga - 2017 - ChemistrySelect - Wiley Online Library

Figure 2 from Rapid degradation of azo dye methyl orange using hollow cobalt nanoparticles. | Semantic Scholar
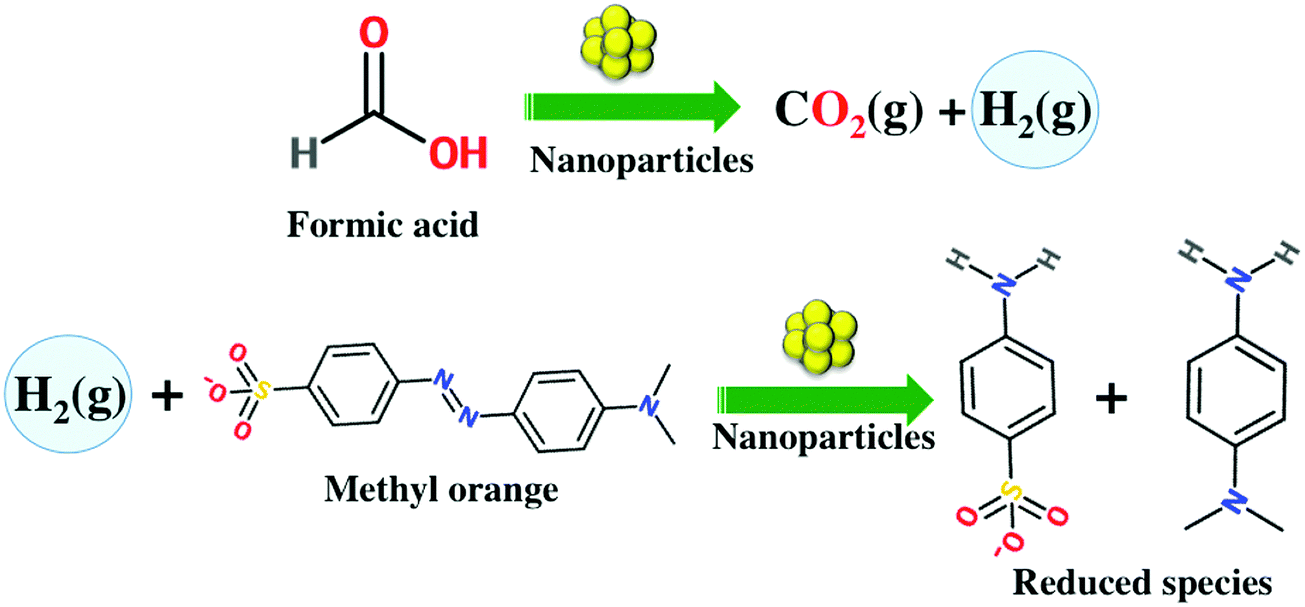
Green synthesis of gold, silver, platinum, and palladium nanoparticles reduced and stabilized by sodium rhodizonate and their catalytic reduction of 4 ... - New Journal of Chemistry (RSC Publishing) DOI:10.1039/C8NJ01223G

Green synthesis, characterization and catalytic degradation studies of gold nanoparticles against congo red and methyl orange - ScienceDirect

Polyaniline Supported Palladium Catalyzed Reductive Degradation of Dyes Under Mild Condition | Bentham Science
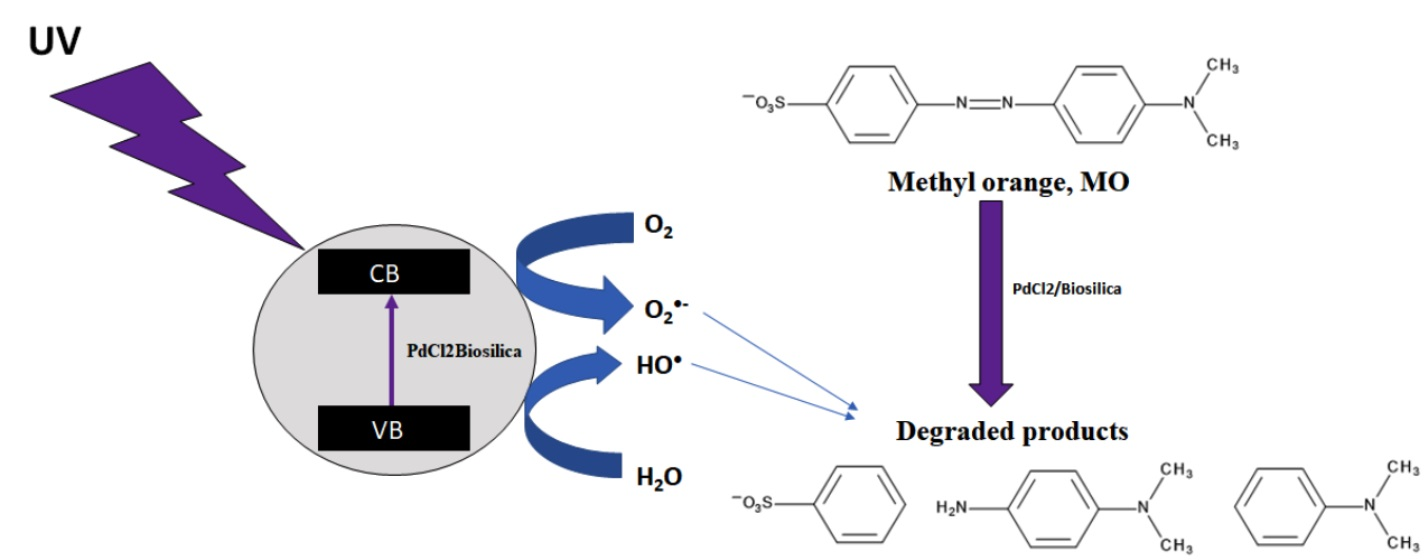
IJMS | Free Full-Text | Diatom Biosilica Doped with Palladium(II) Chloride Nanoparticles as New Efficient Photocatalysts for Methyl Orange Degradation

The specialized twin-solution method for selective Pd(II) ions determination and methyl orange removal - ScienceDirect
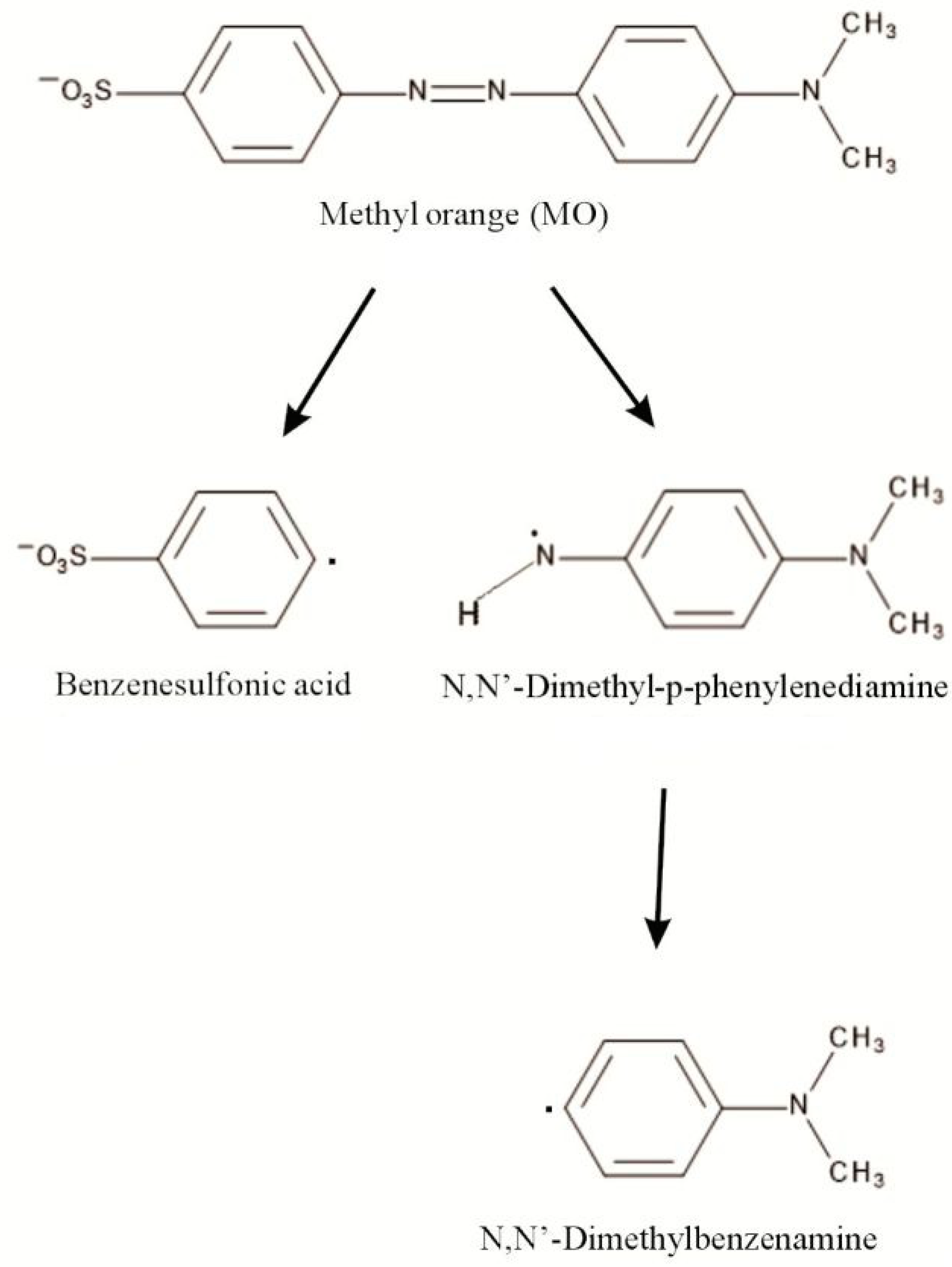
IJMS | Free Full-Text | Diatom Biosilica Doped with Palladium(II) Chloride Nanoparticles as New Efficient Photocatalysts for Methyl Orange Degradation
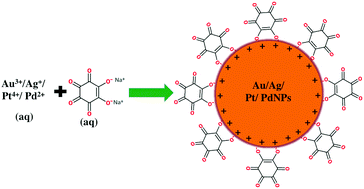
Green synthesis of gold, silver, platinum, and palladium nanoparticles reduced and stabilized by sodium rhodizonate and their catalytic reduction of 4-nitrophenol and methyl orange - New Journal of Chemistry (RSC Publishing)

Degradation mechanism of Methyl Orange by electrochemical process on RuO(x)-PdO/Ti electrode. | Semantic Scholar
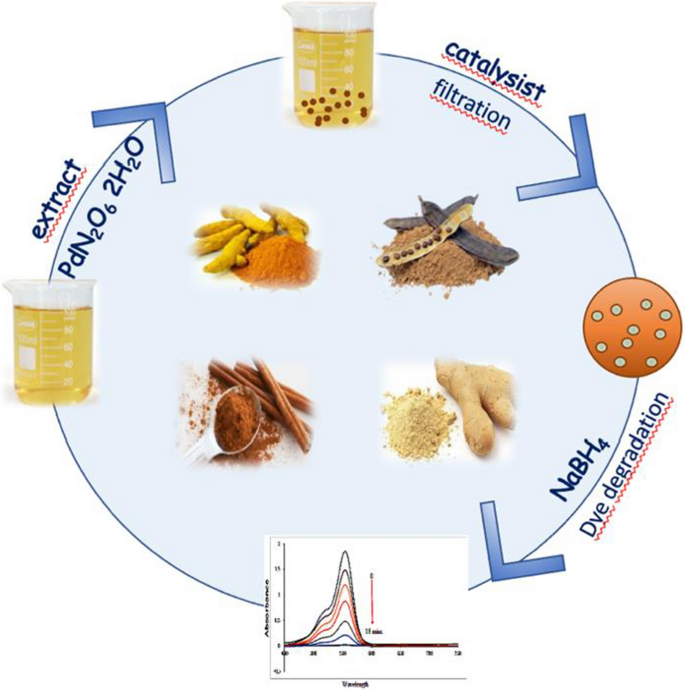
Green synthesis of palladium nanoparticles and investigation of their catalytic activity for methylene blue, methyl orange and rhodamine B degradation by sodium borohydride | SpringerLink

Palladium nanoparticles supported on ionic liquid and glucosamine-modified magnetic iron oxide as a catalyst in reduction reactions | SpringerLink

Acceleration of biotic decolorization and partial mineralization of methyl orange by a photo-assisted n-type semiconductor - ScienceDirect

Chemical structure of methyl orange (MO). Linear formula is C14H14N3NaO3S | Download Scientific Diagram
Tannic acid and palladium-modified magnetite nanoparticles for catalytic degradation of methyl orange - American Chemical Society
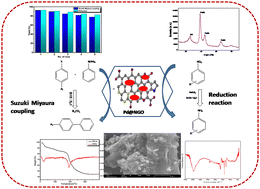
Palladium nanoparticles loaded over sheet-like N-doped graphene oxide: investigation of its catalytic potential in Suzuki coupling, in reduction of nitroarenes and in photodegradation of methyl orange - New Journal of Chemistry (RSC

Degradation of methylene blue and methyl orange by palladium-doped TiO2 photocatalysis for water reuse: Efficiency and degradation pathways - ScienceDirect

Reduction of Sunset Yellow (SY) (A), Methyl Orange (MO) (C), Tartrazine... | Download Scientific Diagram
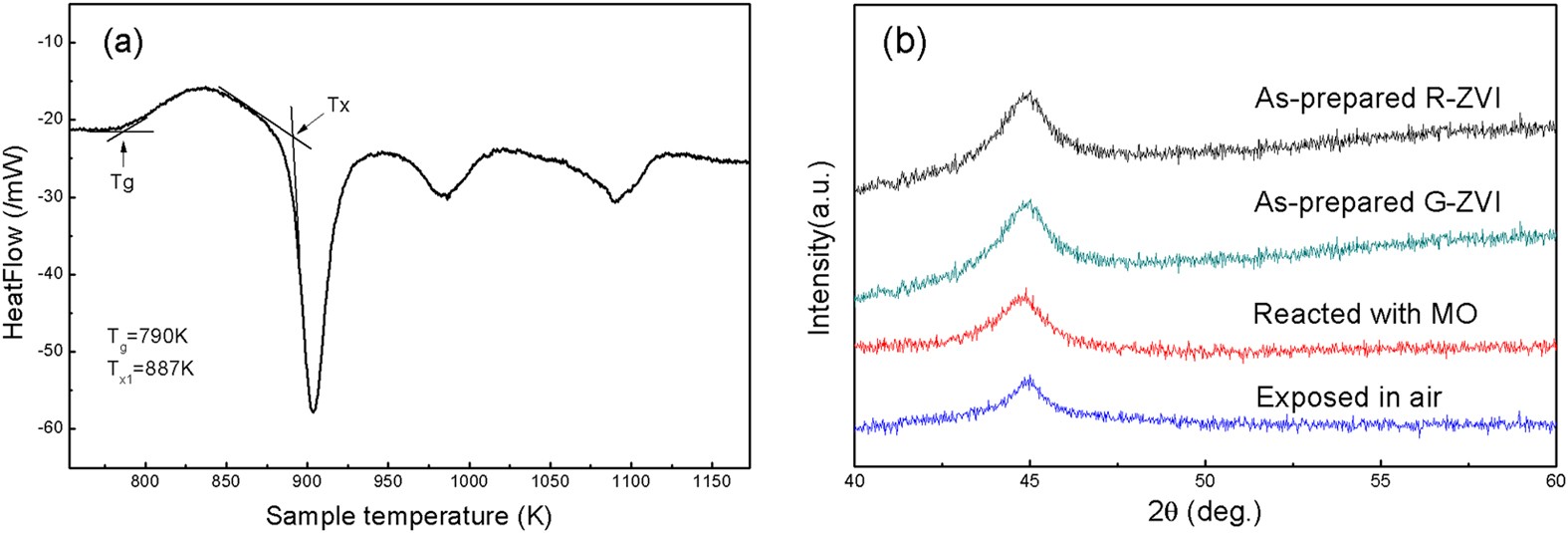
A highly efficient degradation mechanism of methyl orange using Fe-based metallic glass powders | Scientific Reports
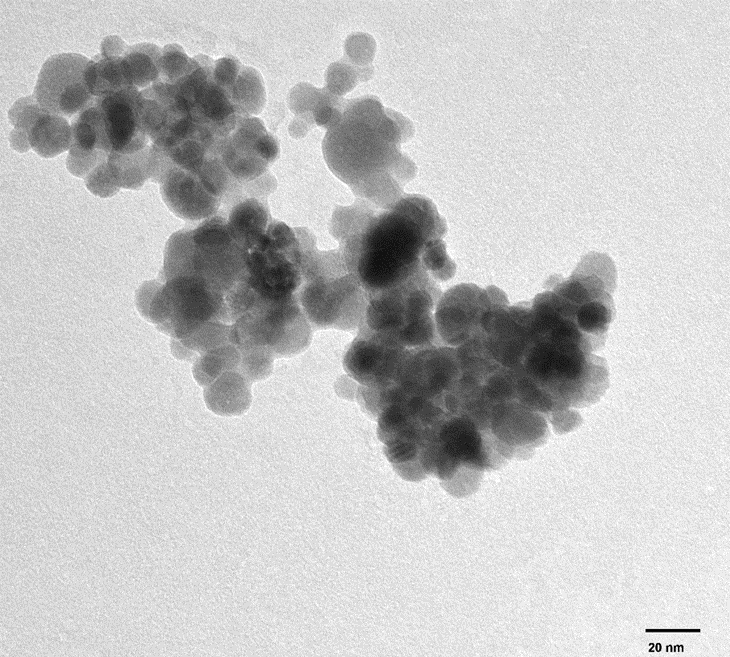
Tannic acid and palladium-modified magnetite nanoparticles for catalytic degradation of methyl orange - American Chemical Society

Pd–Rh Alloyed Nanoparticles on Zeolite Imidazolide Framework-67 for Methyl Orange Degradation | ACS Applied Nano Materials

Degradation mechanism of Methyl Orange by electrochemical process on RuO(x)-PdO/Ti electrode. | Semantic Scholar

Effective Catalytic Reduction of Methyl Orange Catalyzed by the Encapsulated Random Alloy Palladium‐Gold Nanoparticles Dendrimer. - Ilunga - 2017 - ChemistrySelect - Wiley Online Library

Pd–Rh Alloyed Nanoparticles on Zeolite Imidazolide Framework-67 for Methyl Orange Degradation | ACS Applied Nano Materials



